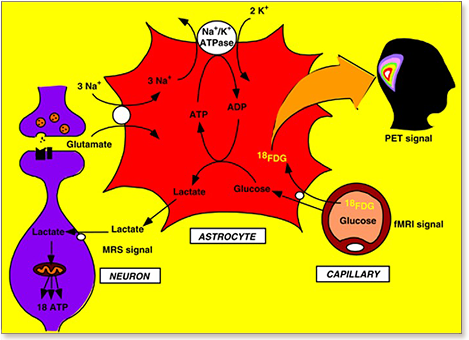
 Since its introduction 16 years ago, the astrocyte-neuron lactate shuttle (ANLS) model has profoundly modified our understanding of neuroenergetics by bringing a cellular and molecular resolution. Praised or disputed, the concept has never ceased to attract attention, leading to critical advances and unexpected insights. Here, we summarize recent experimental evidence further supporting the main tenets of the model. Thus, evidence for distinct metabolic phenotypes between neurons (mainly oxidative) and astrocytes (mainly glycolytic) have been provided by genomics and classical metabolic approaches. Moreover, it has become clear that astrocytes act as a syncytium to distribute energy substrates such as lactate to active neurones. Glycogen, the main energy reserve located in astrocytes, is used as a lactate source to sustain glutamatergic neurotransmission and synaptic plasticity. Lactate is also emerging as a neuroprotective agent as well as a key signal to regulate blood flow. Characterization of monocarboxylate transporter regulation indicates a possible involvement in synaptic plasticity and memory. Finally, several modeling studies captured the implications of such findings for many brain functions. The ANLS model now represents a useful, experimentally based framework to better understand the coupling between neuronal activity and energetics as it relates to neuronal plasticity, neurodegeneration, and functional brain imaging.
Since its introduction 16 years ago, the astrocyte-neuron lactate shuttle (ANLS) model has profoundly modified our understanding of neuroenergetics by bringing a cellular and molecular resolution. Praised or disputed, the concept has never ceased to attract attention, leading to critical advances and unexpected insights. Here, we summarize recent experimental evidence further supporting the main tenets of the model. Thus, evidence for distinct metabolic phenotypes between neurons (mainly oxidative) and astrocytes (mainly glycolytic) have been provided by genomics and classical metabolic approaches. Moreover, it has become clear that astrocytes act as a syncytium to distribute energy substrates such as lactate to active neurones. Glycogen, the main energy reserve located in astrocytes, is used as a lactate source to sustain glutamatergic neurotransmission and synaptic plasticity. Lactate is also emerging as a neuroprotective agent as well as a key signal to regulate blood flow. Characterization of monocarboxylate transporter regulation indicates a possible involvement in synaptic plasticity and memory. Finally, several modeling studies captured the implications of such findings for many brain functions. The ANLS model now represents a useful, experimentally based framework to better understand the coupling between neuronal activity and energetics as it relates to neuronal plasticity, neurodegeneration, and functional brain imaging.