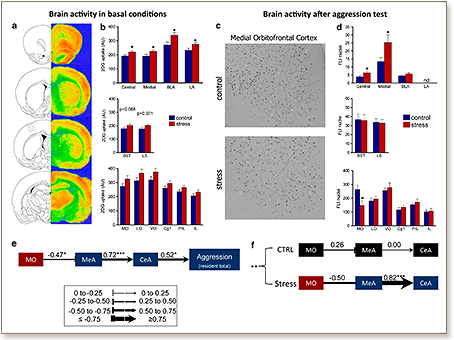
 Although adverse early life experiences have been found to increase lifetime risk to develop violent behaviors, the neurobiological mechanisms underlying these long-term effects remain unclear. We present a novel animal model for pathological aggression induced by peripubertal exposure to stress with face, construct and predictive validity. We show that male rats submitted to fear-induction experiences during the peripubertal period exhibit high and sustained rates of increased aggression at adulthood, even against unthreatening individuals, and increased testosterone/corticosterone ratio. They also exhibit hyperactivity in the amygdala under both basal conditions (evaluated by 2-deoxy-glucose autoradiography) and after a resident-intruder (RI) test (evaluated by c-Fos immunohistochemistry), and hypoactivation of the medial orbitofrontal (MO) cortex after the social challenge. Alterations in the connectivity between the orbitofrontal cortex and the amygdala were linked to the aggressive phenotype. Increased and sustained expression levels of the monoamine oxidase A (MAOA) gene were found in the prefrontal cortex but not in the amygdala of peripubertally stressed animals. They were accompanied by increased activatory acetylation of histone H3, but not H4, at the promoter of the MAOA gene. Treatment with an MAOA inhibitor during adulthood reversed the peripuberty stress-induced antisocial behaviors. Beyond the characterization and validation of the model, we present novel data highlighting changes in the serotonergic system in the prefrontal cortex-and pointing at epigenetic control of the MAOA gene-in the establishment of the link between peripubertal stress and later pathological aggression. Our data emphasize the impact of biological factors triggered by peripubertal adverse experiences on the emergence of violent behaviors.
Although adverse early life experiences have been found to increase lifetime risk to develop violent behaviors, the neurobiological mechanisms underlying these long-term effects remain unclear. We present a novel animal model for pathological aggression induced by peripubertal exposure to stress with face, construct and predictive validity. We show that male rats submitted to fear-induction experiences during the peripubertal period exhibit high and sustained rates of increased aggression at adulthood, even against unthreatening individuals, and increased testosterone/corticosterone ratio. They also exhibit hyperactivity in the amygdala under both basal conditions (evaluated by 2-deoxy-glucose autoradiography) and after a resident-intruder (RI) test (evaluated by c-Fos immunohistochemistry), and hypoactivation of the medial orbitofrontal (MO) cortex after the social challenge. Alterations in the connectivity between the orbitofrontal cortex and the amygdala were linked to the aggressive phenotype. Increased and sustained expression levels of the monoamine oxidase A (MAOA) gene were found in the prefrontal cortex but not in the amygdala of peripubertally stressed animals. They were accompanied by increased activatory acetylation of histone H3, but not H4, at the promoter of the MAOA gene. Treatment with an MAOA inhibitor during adulthood reversed the peripuberty stress-induced antisocial behaviors. Beyond the characterization and validation of the model, we present novel data highlighting changes in the serotonergic system in the prefrontal cortex-and pointing at epigenetic control of the MAOA gene-in the establishment of the link between peripubertal stress and later pathological aggression. Our data emphasize the impact of biological factors triggered by peripubertal adverse experiences on the emergence of violent behaviors.