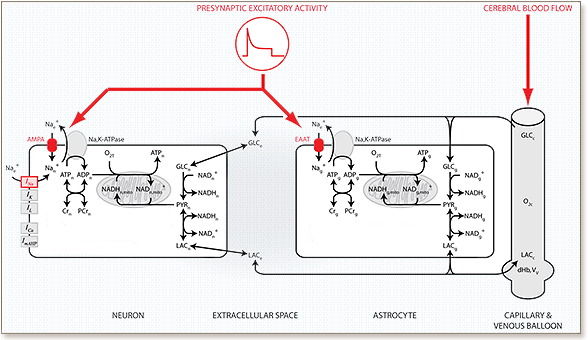
 Glucose is the main energy substrate in the adult brain under normal conditions. Accumulating evidence, however, indicates that lactate produced in astrocytes (a type of glial cell) can also fuel neuronal activity. The quantitative aspects of this so-called astrocyte-neuron lactate shuttle (ANLS) are still debated. To address this question, we developed a detailed biophysical model of the brain’s metabolic interactions. Our model integrates three modeling approaches, the Buxton-Wang model of vascular dynamics, the Hodgkin-Huxley formulation of neuronal membrane excitability and a biophysical model of metabolic pathways. This approach provides a template for large-scale simulations of the neuron-glia-vasculature (NGV) ensemble, and for the first time integrates the respective timescales at which energy metabolism and neuronal excitability occur. The model is constrained by relative neuronal and astrocytic oxygen and glucose utilization, by the concentration of metabolites at rest and by the temporal dynamics of NADH upon activation. These constraints produced four observations. First, a transfer of lactate from astrocytes to neurons emerged in response to activity. Second, constrained by activity-dependent NADH transients, neuronal oxidative metabolism increased first upon activation with a subsequent delayed astrocytic glycolysis increase. Third, the model correctly predicted the dynamics of extracellular lactate and oxygen as observed in vivo in rats. Fourth, the model correctly predicted the temporal dynamics of tissue lactate, of tissue glucose and oxygen consumption, and of the BOLD signal as reported in human studies. These findings not only support the ANLS hypothesis but also provide a quantitative mathematical description of the metabolic activation in neurons and glial cells, as well as of the macroscopic measurements obtained during brain imaging.
Glucose is the main energy substrate in the adult brain under normal conditions. Accumulating evidence, however, indicates that lactate produced in astrocytes (a type of glial cell) can also fuel neuronal activity. The quantitative aspects of this so-called astrocyte-neuron lactate shuttle (ANLS) are still debated. To address this question, we developed a detailed biophysical model of the brain’s metabolic interactions. Our model integrates three modeling approaches, the Buxton-Wang model of vascular dynamics, the Hodgkin-Huxley formulation of neuronal membrane excitability and a biophysical model of metabolic pathways. This approach provides a template for large-scale simulations of the neuron-glia-vasculature (NGV) ensemble, and for the first time integrates the respective timescales at which energy metabolism and neuronal excitability occur. The model is constrained by relative neuronal and astrocytic oxygen and glucose utilization, by the concentration of metabolites at rest and by the temporal dynamics of NADH upon activation. These constraints produced four observations. First, a transfer of lactate from astrocytes to neurons emerged in response to activity. Second, constrained by activity-dependent NADH transients, neuronal oxidative metabolism increased first upon activation with a subsequent delayed astrocytic glycolysis increase. Third, the model correctly predicted the dynamics of extracellular lactate and oxygen as observed in vivo in rats. Fourth, the model correctly predicted the temporal dynamics of tissue lactate, of tissue glucose and oxygen consumption, and of the BOLD signal as reported in human studies. These findings not only support the ANLS hypothesis but also provide a quantitative mathematical description of the metabolic activation in neurons and glial cells, as well as of the macroscopic measurements obtained during brain imaging.