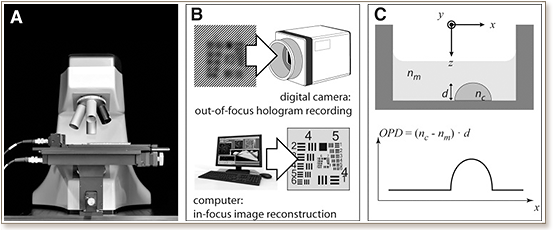
 We introduce a label-free technology based on digital holographic microscopy (DHM) with applicability for screening by imaging, and we demonstrate its capability for cytotoxicity assessment using mammalian living cells. For this first high content screening compatible application, we automatized a digital holographic microscope for image acquisition of cells using commercially available 96-well plates. Data generated through both label-free DHM imaging and fluorescence-based methods were in good agreement for cell viability identification and a Z′-factor close to 0.9 was determined, validating the robustness of DHM assay for phenotypic screening. Further, an excellent correlation was obtained between experimental cytotoxicity dose-response curves and known IC50 values for different toxic compounds. For comparable results, DHM has the major advantages of being label free and close to an order of magnitude faster than automated standard fluorescence microscopy.
We introduce a label-free technology based on digital holographic microscopy (DHM) with applicability for screening by imaging, and we demonstrate its capability for cytotoxicity assessment using mammalian living cells. For this first high content screening compatible application, we automatized a digital holographic microscope for image acquisition of cells using commercially available 96-well plates. Data generated through both label-free DHM imaging and fluorescence-based methods were in good agreement for cell viability identification and a Z′-factor close to 0.9 was determined, validating the robustness of DHM assay for phenotypic screening. Further, an excellent correlation was obtained between experimental cytotoxicity dose-response curves and known IC50 values for different toxic compounds. For comparable results, DHM has the major advantages of being label free and close to an order of magnitude faster than automated standard fluorescence microscopy.