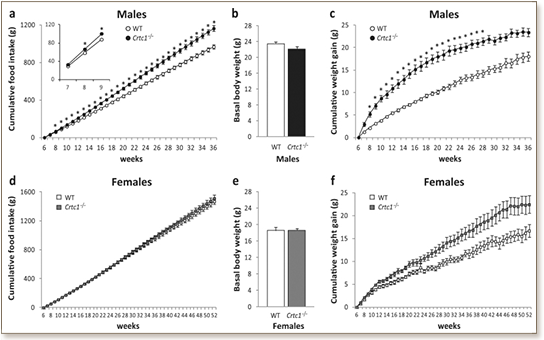
 Obesity and depression are major public health concerns, and there is increasing evidence that they share etiological mechanisms. CREB-regulated transcription coactivator 1 (CRTC1) participates in neurobiological pathways involved in both mood and energy balance regulation. Crtc1 -/- mice rapidly develop a depressive-like and obese phenotype in early adulthood, and are therefore a relevant animal model to explore possible common mechanisms underlying mood disorders and obesity. Here, the obese phenotype of male and female Crtc1 -/- mice was further characterized by investigating CRTC1's role in the homeostatic and hedonic regulation of food intake, as well as its influence on daily locomotor activity. Crtc1 -/- mice showed a strong gender difference in the homeostatic regulation of energy balance. Mutant males were hyperphagic and rapidly developed obesity on normal chow diet, whereas Crtc1 -/- females exhibited mild late-onset obesity without hyperphagia. Overeating of mutant males was accompanied by alterations in the expression of several orexigenic and anorexigenic hypothalamic genes, thus confirming a key role of CRTC1 in the central regulation of food intake. No alteration in preference and conditioned response for saccharine was observed in Crtc1 -/- mice, suggesting that mutant males' hyperphagia was not due to an altered hedonic regulation of food intake. Intriguingly, mutant males exhibited a hyperphagic behavior only during the resting (diurnal) phase of the light cycle. This abnormal feeding behavior was associated with a higher diurnal locomotor activity indicating that the lack of CRTC1 may affect circadian rhythmicity. Collectively, these findings highlight the male-specific involvement of CRTC1 in the central control of energy balance and circadian locomotor activity.
Obesity and depression are major public health concerns, and there is increasing evidence that they share etiological mechanisms. CREB-regulated transcription coactivator 1 (CRTC1) participates in neurobiological pathways involved in both mood and energy balance regulation. Crtc1 -/- mice rapidly develop a depressive-like and obese phenotype in early adulthood, and are therefore a relevant animal model to explore possible common mechanisms underlying mood disorders and obesity. Here, the obese phenotype of male and female Crtc1 -/- mice was further characterized by investigating CRTC1's role in the homeostatic and hedonic regulation of food intake, as well as its influence on daily locomotor activity. Crtc1 -/- mice showed a strong gender difference in the homeostatic regulation of energy balance. Mutant males were hyperphagic and rapidly developed obesity on normal chow diet, whereas Crtc1 -/- females exhibited mild late-onset obesity without hyperphagia. Overeating of mutant males was accompanied by alterations in the expression of several orexigenic and anorexigenic hypothalamic genes, thus confirming a key role of CRTC1 in the central regulation of food intake. No alteration in preference and conditioned response for saccharine was observed in Crtc1 -/- mice, suggesting that mutant males' hyperphagia was not due to an altered hedonic regulation of food intake. Intriguingly, mutant males exhibited a hyperphagic behavior only during the resting (diurnal) phase of the light cycle. This abnormal feeding behavior was associated with a higher diurnal locomotor activity indicating that the lack of CRTC1 may affect circadian rhythmicity. Collectively, these findings highlight the male-specific involvement of CRTC1 in the central control of energy balance and circadian locomotor activity.