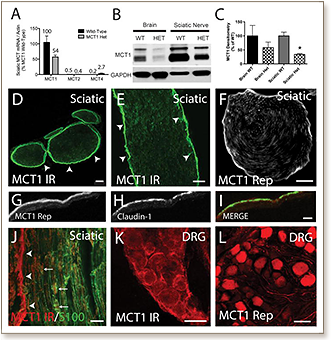
 Peripheral nerve regeneration following injury occurs spontaneously, but many of the processes require metabolic energy. The mechanism of energy supply to axons has not previously been determined. In the central nervous system, monocarboxylate transporter 1 (MCT1), expressed in oligodendroglia, is critical for supplying lactate or other energy metabolites to axons. In the current study, MCT1 is shown to localize within the peripheral nervous system to perineurial cells, dorsal root ganglion neurons, and Schwann cells by MCT1 immunofluorescence in wild-type mice and tdTomato fluorescence in MCT1 BAC reporter mice. To investigate whether MCT1 is necessary for peripheral nerve regeneration, sciatic nerves of MCT1 heterozygous null mice are crushed and peripheral nerve regeneration was quantified electrophysiologically and anatomically. Compound muscle action potential (CMAP) recovery is delayed from a median of 21. days in wild-type mice to greater than 38. days in MCT1 heterozygote null mice. In fact, half of the MCT1 heterozygote null mice have no recovery of CMAP at 42. days, while all of the wild-type mice recovered. In addition, muscle fibers remain 40% more atrophic and neuromuscular junctions 40% moredenervated at 42. days post-crush in the MCT1 heterozygote null mice than wild-type mice. The delay in nerve regeneration is not only in motor axons, as the number of regenerated axons in thesural sensory nerve of MCT1 heterozygote null mice at 4. weeks andtibial mixed sensory and motor nerve at 3. weeks is also significantly reduced compared to wild-type mice. This delay in regeneration may be partly due to failed Schwann cell function, as there is reduced early phagocytosis of myelin debris and remyelination of axon segments. These data for the first time demonstrate that MCT1 is critical for regeneration of both sensory and motor axons in mice following sciatic nerve crush.
Peripheral nerve regeneration following injury occurs spontaneously, but many of the processes require metabolic energy. The mechanism of energy supply to axons has not previously been determined. In the central nervous system, monocarboxylate transporter 1 (MCT1), expressed in oligodendroglia, is critical for supplying lactate or other energy metabolites to axons. In the current study, MCT1 is shown to localize within the peripheral nervous system to perineurial cells, dorsal root ganglion neurons, and Schwann cells by MCT1 immunofluorescence in wild-type mice and tdTomato fluorescence in MCT1 BAC reporter mice. To investigate whether MCT1 is necessary for peripheral nerve regeneration, sciatic nerves of MCT1 heterozygous null mice are crushed and peripheral nerve regeneration was quantified electrophysiologically and anatomically. Compound muscle action potential (CMAP) recovery is delayed from a median of 21. days in wild-type mice to greater than 38. days in MCT1 heterozygote null mice. In fact, half of the MCT1 heterozygote null mice have no recovery of CMAP at 42. days, while all of the wild-type mice recovered. In addition, muscle fibers remain 40% more atrophic and neuromuscular junctions 40% moredenervated at 42. days post-crush in the MCT1 heterozygote null mice than wild-type mice. The delay in nerve regeneration is not only in motor axons, as the number of regenerated axons in thesural sensory nerve of MCT1 heterozygote null mice at 4. weeks andtibial mixed sensory and motor nerve at 3. weeks is also significantly reduced compared to wild-type mice. This delay in regeneration may be partly due to failed Schwann cell function, as there is reduced early phagocytosis of myelin debris and remyelination of axon segments. These data for the first time demonstrate that MCT1 is critical for regeneration of both sensory and motor axons in mice following sciatic nerve crush.