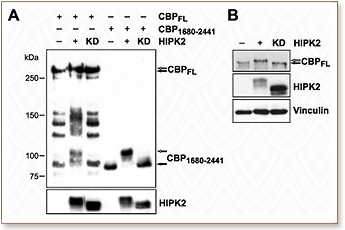
 CREB-binding protein (CBP) and p300 are transcriptional coactivators involved in numerous biological processes that affect cell growth, transformation, differentiation, and development. In this study, we provide evidence of the involvement of homeodomain-interacting protein kinase 2 (HIPK2) in the regulation of CBP activity. We show that HIPK2 interacts with and phosphorylates several regions of CBP. We demonstrate that serines 2361, 2363, 2371, 2376, and 2381 are responsible for the HIPK2-induced mobility shift of CBP C-terminal activation domain. Moreover, we show that HIPK2 strongly potentiates the transcriptional activity of CBP. However, our data suggest that HIPK2 activates CBP mainly by counteracting the repressive action of cell cycle regulatory domain 1 (CRD1), located between amino acids 977 and 1076, independently of CBP phosphorylation. Our findings thus highlight a complex regulation of CBP activity by HIPK2, which might be relevant for the control of specific sets of target genes involved in cellular proliferation, differentiation and apoptosis.
CREB-binding protein (CBP) and p300 are transcriptional coactivators involved in numerous biological processes that affect cell growth, transformation, differentiation, and development. In this study, we provide evidence of the involvement of homeodomain-interacting protein kinase 2 (HIPK2) in the regulation of CBP activity. We show that HIPK2 interacts with and phosphorylates several regions of CBP. We demonstrate that serines 2361, 2363, 2371, 2376, and 2381 are responsible for the HIPK2-induced mobility shift of CBP C-terminal activation domain. Moreover, we show that HIPK2 strongly potentiates the transcriptional activity of CBP. However, our data suggest that HIPK2 activates CBP mainly by counteracting the repressive action of cell cycle regulatory domain 1 (CRD1), located between amino acids 977 and 1076, independently of CBP phosphorylation. Our findings thus highlight a complex regulation of CBP activity by HIPK2, which might be relevant for the control of specific sets of target genes involved in cellular proliferation, differentiation and apoptosis.